As discussed
elsewhere, stem cells or cells derived from them will inevitably play a
key role in restoring function after spinal cord injury (SCI). Since
funding some of the first SCI-focused stem-cell research as PVA’s
research director in the mid-1990’s, I’ve been amazed how many programs
have emerged throughout the world which transplant stem cells from
various sources into individuals with SCI.
However, to fully
appreciate their healing ability, understand that the regenerative
impact of these cells is not just from those externally transplanted
into the patient. From conception until death, they are the cells of
renewal and regeneration inherent within us all.
Stem and progenitor cells are found in most
tissues, including the central nervous system (i.e., brain & spinal
cord). Sometimes, they are involved in ongoing tissue maintenance, such
as the bone-marrow’s production of blood-cell-replenishing stem cells;
in other tissues, they are quiescent and need to be coaxed into action
by appropriate stimuli. In the case of SCI, injury may mobilize dormant
spinal-cord stem-cells into action.
The function of our endogenous stem cells can be
positively or negatively influenced by numerous therapies. For example,
acupuncture and hyperbaric oxygen therapy - both of which have been used
to treat SCI – have been claimed to stimulate their expression. In
contrast, the focus of this article, chemotherapy may be more toxic to
CNS stem cells than the targeted cancer cells. As a result, it can
sabotage the much needed and desired CNS-regenerative potential in
individuals with SCI - even long after the chemotherapy.
In brief, stem and progenitor cells encompass a
continuum of cell types that transform into our end-product tissue. As
our CNS develops, embryonic stem cells generate more specialized
tissue-specific neural stem cells. In turn, these tissue-specific stem
cells can differentiate into neuron- or glial-restricted precursor
cells, the former with the potential to generate neurons and the latter
into CNS support cells called oligodendrocytes and astrocytes.
Especially important for this discussion, oligodendrocytes generate the
insulating myelin sheaths that enwrap axons and are needed for signal
transmission. These distinctions between different cell types are
important because chemotherapy is more toxic to some of the precursor
cell populations than others.
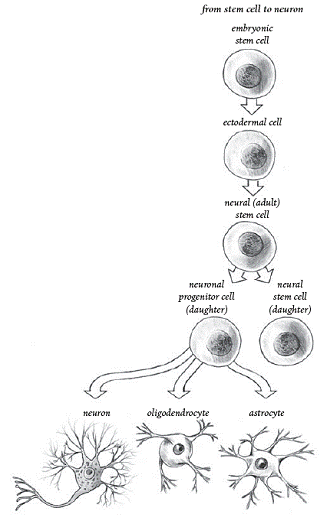
Stem-Cell
Differentiation (From Stem Cell Now, CH Scott, 2005)
Chemotherapy
More than half of cancer patients receive
chemotherapy. Although the research discussed below challenges the
assumption, chemotherapeutic agents have been generally thought to
target rapidly growing, dividing cells, like cancer cells.
Because chemotherapy is administered systemically,
it eradicates cancer cells that have spread throughout the body, but, as
a result, the whole body is vulnerable to potential adverse side
effects. Although the blood-brain barrier may limit somewhat their
infusion into the CNS, cancer drugs do, nevertheless, cross this
barrier, often producing persistent, long-term cognitive deficits. It
has been thought that chemotherapy especially affects brain areas
associated with learning and memory, such as the stem-cell-rich
hippocampus, but there is also substantial evidence of damage to
myelinated regions of the brain (white matter).
Many studies have documented chemotherapy’s
neurotoxicity, especially with high-incidence cancers. For example, 50%
of breast-cancer patients may have cognitive impairments a year after
chemotherapy was stopped. Even 3-6 years later, breast-cancer survivors
have more auditory-processing dysfunction than age-matched controls.
Chemotherapy & CNS Stem Cells
Dr. Mark Noble and his University of Rochester (NY)
colleagues have recently carried out ground-breaking studies that help
us understand more clearly chemotherapy’s neurotoxicity. These studies
have evaluated the impact of various commonly used cancer drugs on CNS
progenitor cells grown in culture (in vitro) and in mice (in
vivo). The cell-culture studies indicated that clinically relevant
levels of commonly used drugs were more lethal to CNS-progenitor cells
than they were for a variety of cancer cell types.

The progenitor cells that evolved into
myelin-producing oligodendrocytes (see illustration) were especially
sensitive to the toxic effects of chemotherapy. In addition,
non-dividing oligodendrocytes themselves were also particularly
vulnerable, a finding which challenges the widely held belief that
chemotherapy only targets dividing cells. Although less sensitive to
chemotherapy than oligodendrocytes, astrocytes (the other key glial
support cell) were still as susceptible as the targeted cancer cells.
Chemotherapy also changed the composition of
surviving cells. Specifically, the oligodendrocyte precursor cells would
not renew themselves through cell division but would differentiate into
oligodendrocytes. The investigators stated “Such a loss of dividing
cells would compromise the ability of dividing progenitor cells to
contribute to repair processes, and could also contribute to
long-term or delayed toxicity reactions.”
Confirming these results, progenitor cells and
oligodendrocytes were compromised in vivo when mice were
systemically given these cancer drugs. Short-term systemic
administration of one of these drugs was shown to cause “both acute CNS
damage and …progressively worsening damage to myelinated tracts of the
CNS…” Consistent with the observations in breast-cancer survivors, this
damage correlated with a delayed loss of function in the mice as
measured by auditory dysfunction.
What about Radiation?
Although beyond the scope of this article, evidence
also suggests that neural precursor cells are extremely sensitive to
radiation, the other twin pillar of cancer treatment. Once again
challenging the assumption that therapy preferentially targets
proliferating cells, radiation is especially lethal to quiescent,
non-dividing neural precursor cells.
Conclusion
Noble et al stated that the “prevalence of cancer
in the world’s populations means that the total number of individuals
for whom adverse neurological changes are associated with cancer
treatment is as great as for the more widely recognized neurological
syndromes.” In other words, the leading cause of neurological
dysfunction may not be disease (e.g., MS) or injury (SCI or head injury)
but that brought about by medicine (i.e., iatrogenic).
Although chemotherapy’s risk-benefit tradeoffs have
always challenged the physician’s pledge to first do no harm, this is an
especially sobering assessment that underscores the need to develop new
treatment options. In addition, chemotherapeutic agents are increasingly
used to treat individuals with autoimmune disease, including MS. It is
thus essential to know whether chemotherapy has adverse effects on
patients with MS receiving such treatment.
On the positive side, Noble’s pioneering work
provides invaluable insights on how we can assess such options with
respect to their ability to preserve the body’s neurological
regenerative potential.
KEY REFERENCES
1)
Meyers CA. How chemotherapy damages the central nervous system.
J Biol 2008; 7.
2)
Dietrich J, et al. CNS progenitor cells and oligodendrocytes are
targets of chemotherapeutic agents in vitro and in vivo. J Biol
2006; 5.
3)
Han R, et al. Systemic 5-flurouracil treatment causes a syndrome
of delayed myelin destruction in the central nervous system. J Biol
2008; 7.
4)
Encinas, et al. Quiescent adult neural stem cells are
exceptionally sensitive to cosmic radiation. Exp Neurol 2008; 210.
5)
Moss RW, Questioning Chemotherapy. Equinox Press, 2004.
Adapted from article appearing in October, 2008 Paraplegia News (For subscriptions,
call 602-224-0500) or go to
www.pn-magazine.com.
TOP